Ever wondered about the connection between sports, architecture and molecular physics? These three distinctive fields come together when we talk about fullerenes. Fullerenes are a modification of carbon with some very interesting properties. The most outstanding one is their structure: They are hollow spheres made up of several penta- and hexagons, resembling a cage. The most famous fullerene C60 (Fig. 1a) actually looks very similar to the traditional pattern of a soccer ball (Fig. 1b). Fullerenes are named after the American architect Richard Buckminster Fuller who is famous for his constructions of geodesic domes, very similar to the fullerenes structure (Fig. 1c). Therefore fullerenes are commonly referred to as Buckminster Fullerenes or Bucky Balls. Fullerenes can build a unique molecular structure where atoms, molecules or even other small clusters are bound inside of the cage. These molecules are called endohedral molecules, with Ho3N@C80 (Fig. 1d) being an example introduced later in this text.

Endohedral molecules have gained some attention in biochemical research for two reasons. First, they are considered excellent vehicles to transport drug molecules to specific locations and release the cage’s content by an externally triggered mechanism. Second, they could be applied in radiotherapy, since the ability to carry metal atoms inside allows them to release a large amount of electrons which cause very localised cell damage, especially to cancer cells.
One mechanism which is expected to play an important role for these two applications is the so-called intermolecular coulombic decay (ICD, not to be confused with the International Classification of Diseases). In an atom, electrons are bound to the nucleus in so-called shells which layer above each other like an onion (a property they share with ogres). To remove an electron from its shell one has to supply energy to it, the closer to the nucleus the shell is, the more energy is needed. A common way of supplying this energy is to shine high-energetic light onto the atoms, either ultraviolet (UV) or even X-rays. If an electron is removed from an atom, we call the remaining atom an ion. If an inner electron is removed, a vacancy or “free spot” in that shell is created. Such ions are called excited.
Speaking from personal experience, excitement tends to decay quickly (citation needed), which also holds true for ions. Within an ion, an electron from a higher shell “falls down” (decays) into the vacancy of the inner shell. By doing so it has to give up the difference in energy between the two shells. One way to give up its energy is by emitting a photon, meaning shining light. This effect is used for example in neon bulbs. If the two involved shells are far enough from each other, the electron can transfer its energy to another electron which is then removed from its shell. This process is called the Auger effect (Fig. 2a). Within molecules another process can happen: The decaying electron can transfer its energy onto an electron of another atom in the molecule which then gets removed from its shell (Fig. 2b). This is the aforementioned ICD.
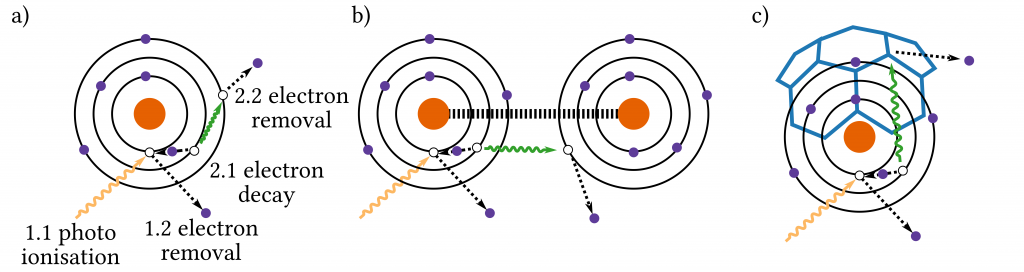
Unfortunately, ICD in endohedral molecules (Fig. 2c) has, even though theoretically predicted, not been discovered. Well, until recently. Dr. Razib Obaid and colleagues set up an experiment at the Advanced Light Source (ALS) in Berkeley, one of the world’s brightest UV and X-ray light source facilities in the world. They used the UV light to radiate the molecule Ho3N@C80 (a molecule consisting of three holmium and one nitrogen atom, trapped in a cage of 80 carbon atoms). The result was the production of ions and electrons, which the researchers measured together with their energy distribution. Additionally, they measured the relation of the particle’s production time. Putting these measurements together, they were able for the first time to demonstrate ICD in endohedral molecules. This required not only a clever experimental setup, but also a lot of theoretical effort. The complexity of the experiment and its analysis derives from the fact that ICD involves multiple atoms with many electrons. This makes the measured spectra resulting from such experiments difficult to disentangle and complicates the assignment of each individual process.
With the first clear observation of ICD in endohedral fullerenes, demonstrating the existence of the proposed mechanism, the researchers have opened the door to further research on the application of the process as a drug delivery system and its influence in the propagation of radiation induced molecular damage in biomolecules.
Dr. Razib Obaid is currently a postdoc at RARAF Radiological Research Accelerator Facility located at the Nevis Laboratory of Columbia University, lead by Dr. David J. Brenner.
Images:
Figure 1b: Derived from Football (soccer ball).svg. (2020, September 23). Wikimedia Commons. Retrieved 23:10, August 30, 2021
Figure 1c: Biosphere, Montreal.jpg. (2020, October 26). Wikimedia Commons,. Retrieved 23:11, August 30, 2021