Pain is an essential sensation for the survival of organisms. It acts as a protective mechanism, signaling potential harm and prompting animals to recognize noxious stimuli for avoiding future harm. For example, individuals unable to feel pain sensations (a rare congenital disease) seem advantaged at first glance; however they are actually at a high risk of unknowingly injuring themselves. While acute pain serves a crucial role in preserving well-being, chronic or persistent pain can severely impact the quality of life. Unfortunately, the mechanisms underlying the transition from acute to chronic pain remains poorly understood, making diagnosis and treatment challenging. Although opioids are commonly used to manage chronic pain, they carry the risk of addiction and interference with normal brain activity. As an alternative, targeting directly the receptors involved in pain pathways, such as the transient receptor potential (TRP) family, offers a promising avenue for developing analgesic therapies.
A recent study (https://www.nature.com/articles/s41467-023-38162-9) led by Dr. Arthur Neuberger, a postdoctoral research scientist in the department of Biochemistry and Molecular Biophysics within Dr. Alexander Sobolevsky’s lab, investigated the structure and inhibition mechanism of human TRPV1 and its interaction with a potential analgesic compound known as SB-366791.
TRPV1, a receptor found on nerve cells, plays a pivotal role in sensing pain and heat. It is a temperature-sensitive TRP ion channel, commonly referred to as the vanilloid receptor 1 or capsaicin receptor (pungent compound from chili pepper). TRPV1 functions as an ion channel involved in pain sensation, becoming activated in response to noxious signals. When activated, TRPV1 opens, leading to a decrease in the electrical resistance of the cell membrane and the subsequent flow of ions. If the strength of the noxious signal and the response of the ion channel are sufficient, a change in the membrane potential occurs, allowing the signal to be transmitted from the peripheral nervous system to the spinal cord and ultimately to the brain (Figure 1).

Figure 1. Overview of the pain-processing pathway. When exposed to noxious stimuli, such as the burning sensation of a flame on the skin or the heat sensation of capsaicin on the tongue, TRPV1 ion channels are activated in the peripheral nervous system. The resulting heat signal is transmitted through nerve fibers and follows spinal pathways until it reaches the brain, where it triggers the generation of a pain response. Figure created using Biorender.
The study, published in Nature Communications, significantly advanced our understanding of the three-dimensional structure of TRPV1, providing crucial insights into its functional properties. The researchers employed advanced techniques, such as cryo-electron microscopy (cryo-EM), to determine the precise arrangement of human TRPV1. Additionally, whole-cell patch clamp electrophysiology (technique that measures the electrical signals as flow of ions across the cell membrane providing information on how the cell responds to certain stimuli or drugs) was used to propose the inhibition of TRPV1 by SB-366791. These cutting-edge techniques provided valuable insights into the architecture and functional characteristics of TRPV1 (Figure 2). Through their investigations, the researchers successfully identified the specific binding site of SB-366791 on the vanilloid site of TRPV1, utilizing cryo-EM and mutagenesis techniques. This discovery suggests that the analgesic compound may exert its effects by selectively inhibiting TRPV1 activity. Furthermore, the study revealed the structural changes that occur within TRPV1 upon binding to SB-366791. These structural alterations affect the conformation of the protein, demonstrating that even in the closed state of TRPV1, SB-366791 can bind and stabilize the closed conformation. This mechanism has the potential to reduce pain signaling. Understanding these structural modifications is important for the development of novel analgesic drugs targeting TRPV1.
Traditionally, pharmacological and physiological investigations of TRPV1 have primarily focused on rodent and squirrel orthologues. However, since the TRPV1 channel plays a central role in almost every aspect of human physiology and disease, including pain and temperature perception, the recent study by Neuberger and colleagues is significant. By specifically examining the human TRPV1 structure and its interaction with SB-366791, this research opens up new possibilities for the development of effective analgesic therapies and sets the stage for future investigations in the field of pain management.
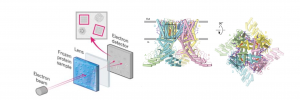
Figure 2. Overview of cryo-electron microscopy (cryo-EM) and the structure of human TRPV1 in complex with the inhibitor SB-366791. In cryo-EM, a beam of electrons is directed at a frozen solution containing the protein of interest. The resulting electrons pass through a lens system, creating a magnified image on a detector. From this image, a three-dimensional model of the protein’s structure is generated. The structure of hTRPV1 in complex with SB-366791 is visualized from two perspectives: parallel to the membrane and extracellularly. In the images, the TRPV1 subunits are color-coded as green, yellow, pink, and blue, while the lipids are represented as purple sticks. (Figure modified from Neuberger et al., 2023)
Reviewed by: Maaike Schilperoort, Giulia Mezzadri and Trang Nguyen