Ever wonder how our muscles work when we move? It all comes down to calcium—a key player, released from storage spaces within our muscle cells called the sarcoplasmic reticulum (SR), that regulates the muscle contraction. The crucial protein allowing calcium release is the type 1 ryanodine receptor (RyR1), an ion channel of enormous proportions (the largest known so far) that is embedded in the SR membrane.
RyR is more than just a protein; it’s a critical piece of the puzzle in understanding diseases like muscular dystrophy and heart failure. Plus, RyR serves as a potential pharmacological target, opening avenues for therapeutic interventions in muscle-related disorders.
Yet, working with such bulky proteins in its native state – within the cell membrane- is a challenging subject. Typically, the steps needed to resolve the structure of proteins normally involve using detergents and harsh conditions that disrupt the native state of a protein.
However, why is it crucial to comprehend the structure of proteins in their native state?? Because it enables us to mimic the conditions in living cells and gain valuable insights into their physiological relevance, their interaction with other molecules, exploring disease mechanisms, and facilitating drug development, among others.
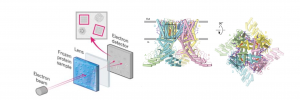
So far, it has been used cryogenic electron microscopy (cryo-EM), an imaging technique that freezing samples and using electrons instead of light to reveal the protein’s intricate 3D structure (i.e., protein’s shape, how its various parts are arranged, and the interactions between its amino acids). While the 3D arrangement of the RyR1 protein had been solved at a very detailed level (referred to as the “High-resolution structure”), the configuration in its native state was still unknown.
In the pursuit to unravel the structure of proteins within native or near-native membranes, a recent breakthrough has provided a high-resolution view of RyR1.
Departing from traditional detergent-based methods used to solubilize and stabilize membrane proteins, Dr Melville Zephan, CUIMC postdoc, and colleagues adopted a gel-filtration approach with on-column detergent removal, they’ve captured RyR1 within liposomes—tiny bubbles mimicking cell membranes (Figure 1).

Figure1. Flow diagram illustrating protein-incorporated liposomes.
RyR1 from rabbit skeletal muscle was homogenized and purified using ion-exchange chromatography, a technique that separates compounds based on their electric charge. Liposomes, which are tiny vesicles made of self-assembly of lipid bilayers in water, were formed using gel filtration. This process helped incorporate RyR1 into liposomes and remove any excess or empty liposomes. (Adapted from Melville Z. Cell Press 2021)
This novel technique maintains a more natural environment, allows to purify membrane proteins and remove detergent molecules in excess, offering a clearer glimpse into how RyR1 functions (Figure 2).

Figure2. RyR1 representation in native and near-native membranes incorporated in liposome after gel filtration. (Adapted from Melville Z. Cell Press 2021)
Their study reveals how RyR1 behaves in its natural environment, forming a network of channels within cell membranes. While their investigation in liposomes gives only a partial view of this complexity (without seeing how molecules interact), it sets the stage for future investigations. This approach, extending beyond RyR, offers a blueprint for studying membrane proteins in native conditions.
In summary, this work successfully captured the intricate structure of RyR1 in liposomes opening doors to a better understanding of muscle physiology and offers a sharper lens for exploring the complex world of membrane proteins.
For more details on this research, refer to the article: “High-resolution structure of the membrane-embedded skeletal muscle ryanodine receptor”.
https://www.sciencedirect.com/science/article/pii/S0969212621002963?via%3Dihub
Reviewed by: Trang Nguyen, Carlos Diaz, Erin Cullen