Have you ever wondered how two eyes can work together to create a single image? This occurs through a complicated mechanism called binocular vision, or stereopsis. When light hits the eye, photosensitive cells called rods and cones translate that light into an electrical signal, which they communicate to retinal ganglion cells (RGCs). RGCs have long axons that transmit visual signals to neurons in the brain. These axons are initially carried from the eye to the brain in a bundle called the optic nerve. Each eye has an optic nerve, and the two optic nerves meet at an intersection called the optic chiasm. At the optic chiasm, some of the RGC axons from each optic nerve cross to the opposite, or contralateral, side of the brain and the rest stay on the same, or ipsilateral, side. This process allows the part of the world that the left eye can see and the part that the right eye can see to overlap in the center, forming one cohesive image.
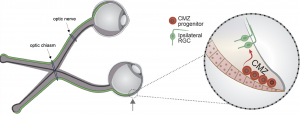
When binocular vision does not develop normally, depth perception can be impaired. This is often the case in albinism, a genetic condition characterized by low levels of melanin that occurs in humans and other mammals. While melanin is most widely known for its contributions to skin color in humans, it also plays an important role in the development of the visual system. The exact nature of this role is still being uncovered. Recent work emerging from a collaboration between the Mason-Dodd lab and the John lab at Columbia University’s Zuckerman Institute, led by Postdoctoral Research Scientist Dr. Nefeli Slavi and Associate Research Scientist Dr. Revathi Balasubramanian, used albino mice to identify why deficits in depth perception might occur in albinism. The study, published in the leading neuroscience journal Neuron, also advanced our understanding of the development of binocular vision generally.
Most RGCs are formed in a location called the neural retina during development, but recent research has uncovered a prominent role for another source of RGCs called the ciliary margin zone (CMZ). The CMZ produces mostly ipsilaterally projecting RGCs, which are present at abnormally low levels in albinism. Based on this existing research, Drs. Slavi and Balasubramanian and their co-authors decided to investigate why these cells are not produced at typical levels in albino mice. First, the group found that there were fewer cells in the actively dividing phases of the cell cycle in the CMZ in albino mice. Further investigation revealed that this deficiency in cell division led to a lower number of ipsilaterally projecting RGCs in these mice. They traced these deficits to decreased expression of a protein called CyclinD2, which is known to encourage cell division. To further investigate the role of CyclinD2 in cell division in the CMZ, the researchers used mice that were pigmented (not albino) but did not express CyclinD2 in the CMZ. These CyclinD2-deficient mice had the same impairments in cell division as the albino mice.
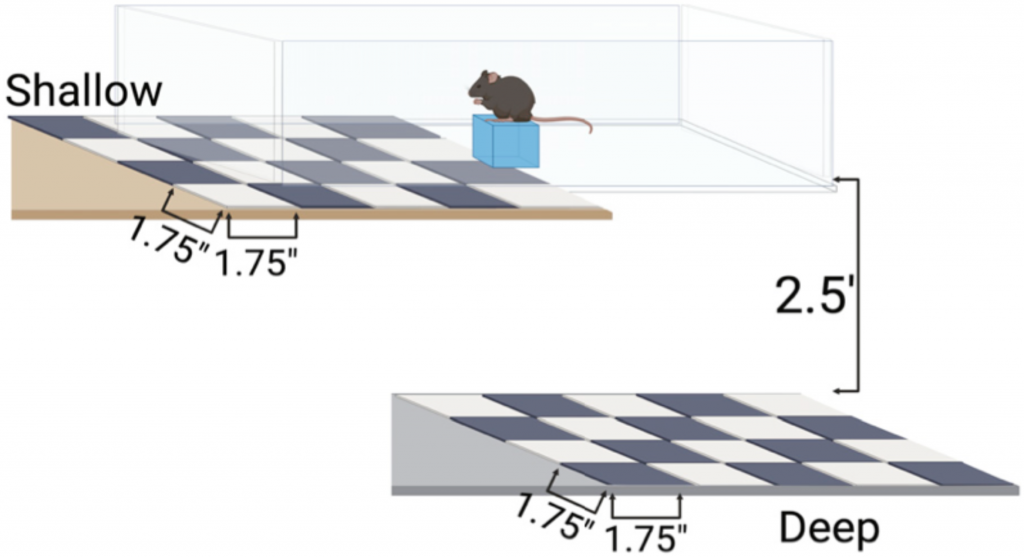
Next, the researchers tested the impact of this impaired cell division on depth perception. They assessed the depth perception of albino and CyclinD2-deficient mice compared to control pigmented mice using an established setup called the visual cliff task. In the task, mice are placed on a narrow platform on a smooth glass surface with a shallow drop below the glass on one side and a far drop on the other side. They assessed each mouse’s depth perception based on how often they chose the shallow, safer side. This task showed that albino and CyclinD2-deficient mice were unable to recognize the difference between the shallow and deep sides, demonstrating impaired depth perception.
Once the researchers discovered that the deficit in the number of ipsilaterally projecting neurons was important for visual function, they turned to attempting to correct it. They found a clue in a set of nearly 25 year old studies, which indicated that CyclinD2 might be able to be increased by activating calcium channels in the neuronal membrane. To test the effect of calcium channel activation on neurogenesis and depth perception, the researchers injected pregnant albino mice with a calcium channel-activating drug called BayK-8644. The drug increased CyclinD2 activity during a critical period for RGC neurogenesis in the mid-gestational mouse pups. In those pups, ipsilateral neurogenesis was restored significantly, though not normalized to the level of the control pigmented mice. When these mice were adults, the visual cliff task was used to assess their depth perception. Remarkably, the increase in CyclinD2 activity and subsequent increase in RGC neurogenesis during gestation dramatically improved binocular vision in the albino mice. However, the drug did not rescue these deficits in CyclinD2-deficient mice, suggesting that the presence of CyclinD2 is necessary for the development of binocular vision.
This paper describes a novel role for the CMZ in the development of the retina and binocular vision, expanding our collective understanding of how the complex mammalian visual system develops. It also identified a role for CyclinD2 in this process, further showing that the presence of CyclinD2 is necessary for the development of binocular vision. These discoveries uncovered new directions for further understanding the development of the visual system and treating vision-related disorders.
Reviewed by: Trang Nguyen and Flavia Dei Zotti