While genomes include the totality of genes that determine an organism’s biological identity, genes can only be a tiny fraction of the genome. In humans, and in many other species, DNA contains multiple diverse regions. One example are transposons, or mobile genetic elements, which are parts of DNA that can move in new places in the genome (Figure 1). The “genomic walks” of transposons are potentially harmful, since when they “jump” within a gene, they trigger mutation and loss of that gene. Cells are constantly evolving new strategies to keep transposons under control. In response, transposons adapt and arm themselves with new strategies to escape cellular control.
Sometimes, a mobilized transposon hijacks additional DNA information that it transfers to the new location with itself. When this additional DNA happens to be a gene providing survival advantage, not only the recipient increases its gene pool and survival capacities but the transposon also increases its chances to propagate. This process of transmission of information between different species is termed horizontal gene transfer and represents a major driver of genome evolution across all domains of life. Transposons have been key players in this process.

Figure 1. Schematic representation of a DNA transposon and its movement between two DNA molecules. Image created with BioRender.com.
Transposons can be considered as reminiscent of ancient viral infections that managed to integrate in a host cell genome but later lost the capacity to completely exit the cell. Bacteria have evolved a fascinating mechanism to remember viruses that they have already encountered. This acquired immunity involves a family of DNA sequences named CRISPR and their associated protein partners Cas. The CRISPR sequences are fragments of DNA derived from different viruses that have infected the bacteria and were integrated in the bacterial genome, creating a footprint of previous infections (Figure 2, upper part). When cells are infected, they use the CRISPR catalog and compare it to the invading DNA, helping it to recognize recurrent viruses and destroy them more rapidly. Cas proteins participate in the degradation of the viral DNA. (Figure 2, lower part).
Advances in research showed that the CRISPR-Cas complexes can be modified to edit genes in different organisms. To do this, part of the complex is changed to lead the Cas protein to a gene of interest, instead towards a viral genome. This system of gene editing has already found numerous important applications ranging from basic biology research to disease treatments and development of new technologies. The discovery of CRISPR-Cas was acknowledged with the 2020 Nobel Prize in Chemistry to Emmanuelle Charpentier and Jennifer Doudna.
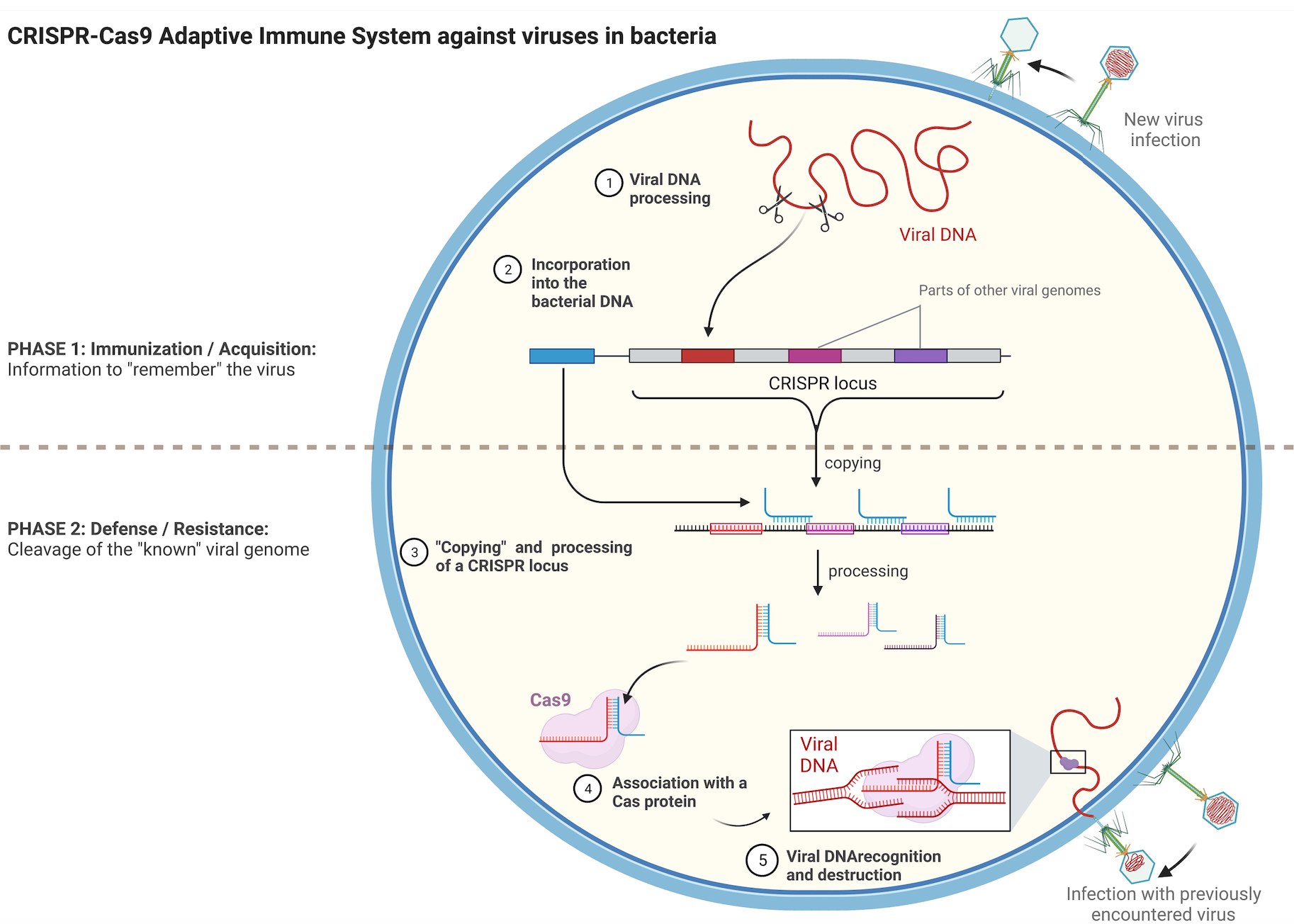
Figure 2. Schematic representation of the CRISPR-Cas9 adaptive immune system of bacteria. Briefly, upon infection the viral DNA is fragmented and part of it is integrated in a special region of the bacterial genome, the CRISPR locus. CRISPR sequences get copied into shorter RNA molecules that carry parts of sequences identical to the sequence of a certain virus. Once copied, these short RNAs form complexes with Cas proteins and serve as “guides” for them towards potential complementary viral DNAs. The viral DNA is destroyed by the Cas protein if it can hybridize with the short RNA from the CRISPR locus. Image created with BioRender.com.
Interestingly, researchers have found an intriguing interconnection between transposons and the CRISPR–Cas defense system. In a striking example a bacterial transposon has hijacked some of the genes of a CRISPR-Cas system and uses those genes for its own propagation in genomes. These transposons are called CRISPR-associated transposons. Such widespread exchange of genes is caused by the never-ending arms race between the transposons and their hosts’ defense systems. In the recent publication of Hoffman and colleagues, five Columbia postdocs Minjoo Kim, Leslie Y. Beh, Jing Wang, Diego R. Gelsinger, and Jerrin Thomas George collaborated and brought significant insight in the functioning of one CRISPR-associated transposon.
In their publication, the authors monitored in detail the formation of this RNA-guided transposon and its associated complexes, which enabled them to resolve distinct protein recruitment events that take place before the integration of the transposon. They also found that even if initially hundreds of non-desired genomic sites are targeted for integration at the end only few of those sites recruit the whole transposon machinery that is required for integration at this genomic location. This discovery offered insights into how the potential target sites in the host genome are identified, screened and approved for integration, allowing the transposon system to be specific.
To advance the understanding of interactions responsible for the assembly of the transposon associated proteins, the authors determined the structure of one of the interacting proteins, named TnsC and found that it is forming rings of seven molecules of TnsC that can pass DNA through the central pore of the ring. This helps to correctly position DNA for the following integration (Figure 3). The resolved molecular structure also allowed to gain clarity on how TnsC mediates the communication between the proteins in this transposon complex that are responsible on the one hand for the targeting and on the other hand for the integration at a specific genomic location. Their results pointed to TnsC as the proofreading checkpoint that ensures the specific selection of genomic sites for transposition.
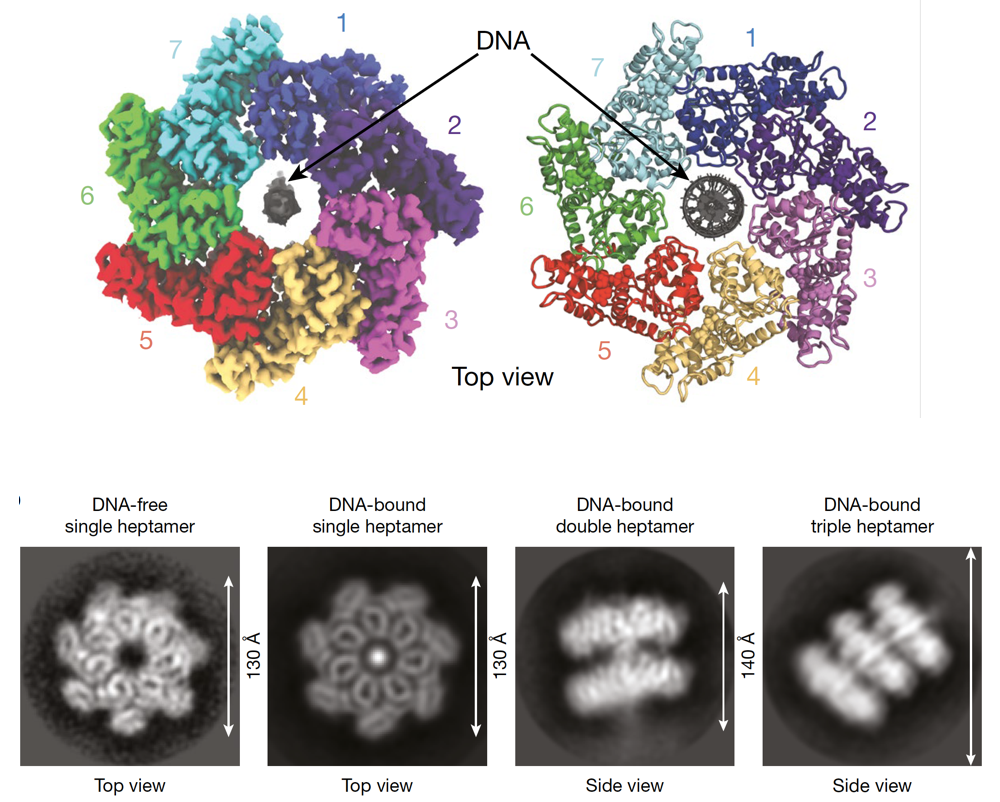
Figure 3. Upper part: Model of the 7 molecules of TnsC forming a ring through which DNA is passed. Lower part: Representative experimental result from Hoffmann et al. showing different configurations and views of the DNA-TnsC complex. Figure adapted from the original publication.
In summary, the paper not only deciphers the molecular specificity of consecutive factor binding to genomic target sites in this interesting process of RNA-guided transposition, but the resolved detailed structure also provides valuable information for the development of future biotechnologies in the field of programmable and specific integration of DNA in desired genomic locations. Such technology differs from the above-described original CRISPR-Cas system currently used for genome editing, because it has the potential to be less mutagenic, as well as because it provides the opportunity to insert much longer pieces of DNA in a desired location. RNA-guided transposases hold tremendous potential for future biotechnological and human therapeutic applications and will without a doubt accelerate novel discoveries. Find out more in the original publication.
Reviewed by: Trang Nguyen & Maaike Schilperoort