Glioblastoma WHO grade IV (GBM) is the most common primary brain tumor in adults. The therapeutic options for this recalcitrant malignancy are very limited with no durable response. A recent research article published in Nature Communications identified how the tumor cells alternate their metabolism to survive using targeted drug treatment in cell lines and mouse models. The project was led by Dr. Nguyen, a postdoctoral research scientist in Dr. Siegelin’s lab at Columbia University.
Most cancer cells produce energy in a less efficient process called “aerobic glycolysis”, consisting of high levels of glucose uptake and generate lactic acid in the cytosol in the presence of abundant oxygen. This classic type of metabolic change provides substrates required for cancer cell proliferation and division, which is involved in tumor growth, metastatic progression and long-term survival. Dr. Siegelin’s laboratory at the Department of Pathology and Cell Biology at Columbia University Medical Center focuses on targeting cell metabolism and the epigenome for brain tumor therapy by using clinical validated drugs to suppress the tumor growth in glioblastoma. In this study, the authors used Alisertib (MLN8237), a clinically validated highly specific Aurora A inhibitor to target brain tumors. Aurora A kinases (AURKAs) are important for the proliferation and growth of solid tumors, including glioblastomas. Here, the authors found that Aurora A simultaneously interacts with both c-Myc (MYC Proto-Oncogene) and GSK3β (Glycogen Synthase Kinase 3 Beta). AURKA stabilizes the c-Myc protein and promotes cell growth. AURKA inhibitor, displayed substantial downregulation of the c-Myc protein. c-Myc (MYC) is an oncogenic transcription factor that facilitates tumor proliferation in part through the regulation of metabolism. Inhibition of Aurora A will lead to a degradation of c-Myc mediated by GSK3β. The authors also found that inhibition of Aurora kinase A suppressed the glycolysis signaling pathway in glioblastoma cells which was related to the degradation of c-Myc protein (Figure 1).
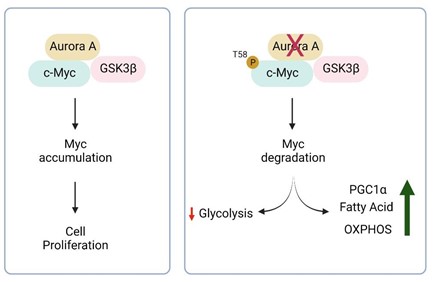
Figure 1: In the cells, Aurora A binds to c-Myc and facilitates cell proliferation. Inhibition of Aurora A will stop the cell from generating energy through glycolysis, a metabolic pathway that converts glucose to energy in cytosol, due to the degradation of c-Myc. c-Myc is marked for degradation by its phosphorylation at position T58 mediated by GSK3β. To survive, the cells start to use different pathways to generate energy by e.g. burning fat or proteins. Figures created with Biorender.com.
In addition to the acute treatment, it is important to understand how tumor cells acquire mechanisms to escape from chemotherapy following constant exposure to a drug and identify means to prevent this phenomenon from occurring. The research group generated drug-resistant cells by culturing them in the presence of alisertib for two weeks. These cells acquire partial resistance to alisertib and display a hyper-oxidative phenotype with an increase in the size of mitochondria with a tubulated shape.
The chronic Aurora A inhibited cells were analyzed for the expression of genes that were modified after constantly applying the same dose of alisertib for a long term period. Researchers found that resistance alisertib cells activate oxidative metabolism and fatty acid oxidation such as an increase in the generation of fatty acid proteins. These observations prompted them to test the hypothesis that alisertib along with fatty acid oxidation inhibitors such as etomoxir will reduce the cellular viability of glioblastoma cells. Etomoxir is a clinically validated drug that binds and blocks the mitochondrial fatty acid transporter. The authors found that the combination treatment of alisertib and etomoxir resulted in enhanced cell death as compared to single treatments and vehicles.
Given the significant promise of in vitro studies, the researchers extended their study in vivo by injecting the patient-derived glioblastoma cells acquired from the patient brain tumors in immunocompromised mice. Such model systems are currently considered to be in closest resemblance to the patient scenario. They found that the combination treatment extended animal survival significantly longer as compared to single treatment with alisertib or etomoxir, suggesting potential clinical efficacy. Taken together, these data suggest that simultaneous targeting of oxidative metabolism and Aurora A inhibition might be a potential novel therapy against deadliest cancers.
Article reviewed by: Maaike Schilperoort, Vikas Malik, Molly Scott, Pei-Yin Shih and Samantha.