Have you ever realized that you remember experiences associated with strong emotions more vividly? For example, you probably remember what you ate at your (or a close friend’s) wedding, but not last Tuesday. However, these persistent memories are not always pleasant. People exposed to actual or threatened death, serious injury, or sexual violence can develop Post-Traumatic Stress Disorder (PTSD), which involves recurring memories or dreams of the traumatic event, bodily reactions to reminders and active avoidance of those reminders. Treatment for PTSD combines psychotherapy and medication, and it aims at enabling the person to understand their trauma and detach the triggers from the responses.
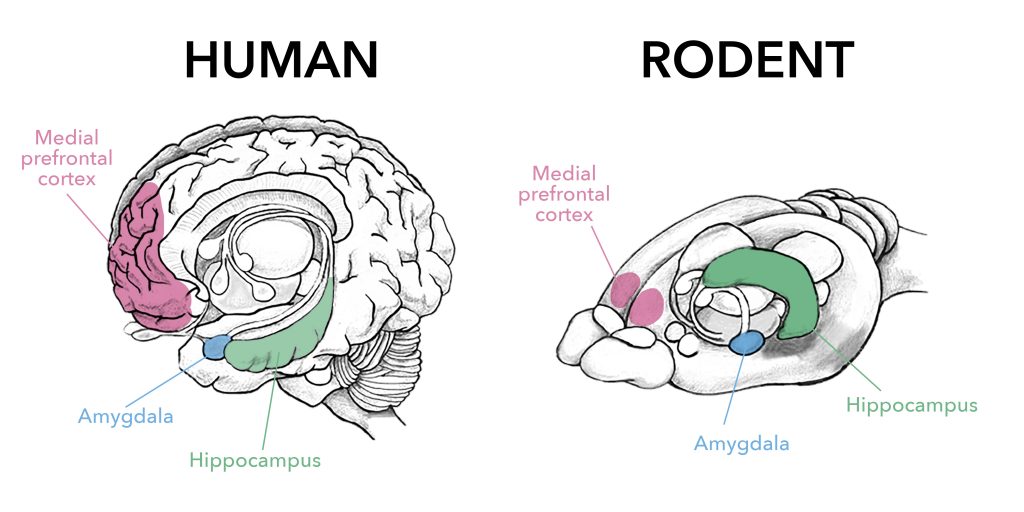
The area in your brain responsible for the formation of such emotional memories is called the amygdala (from the Greek word for almond, due to its shape, Fig. 1). It can modify the way it will respond to similar stimuli in the future, and it can also affect how other brain areas, like the medial prefrontal cortex or the hippocampus, do as well. This ability to change and adapt is called plasticity, and it can start with something as “simple” as a synaptic connection becoming stronger or weaker. There are higher levels of plasticity, though. If changes alter the potential response of a region to a future challenge, this plasticity of plasticity is called metaplasticity.
In the recent review “Intra-Amygdala Metaplasticity Modulation of Fear Extinction Learning”, CUIMC postdoc Dr. Rinki Saha and colleagues provide a comprehensive account of recent literature on metaplasticity in the amygdala in the context of fear conditioning, and how it may lead to plasticity in other connected brain regions.
Fear conditioning is a classic rodent model in neuroscience research that allows scientists to study the mechanisms that lead to associations between neutral stimuli and unpleasant stimuli. The general experimental layout is as follows: first, a neutral stimulus (a light or a tone, for example) is consistently paired to precede an aversive stimulus (like an electric foot shock). After this exposure, animals learn that the neutral stimulus (called conditioned stimulus) predicts the aversive one (called unconditioned stimulus) and they develop a fear response which they perform right after the neutral stimulus (life freezing in place). The experiment can continue to study how they learn to dissociate them once the stimuli stop being paired. For this second part, called fear extinction learning, the neutral stimulus is presented by itself (without pairing it to the aversive one), and researchers measure the time it takes the animal to stop performing the fear response.
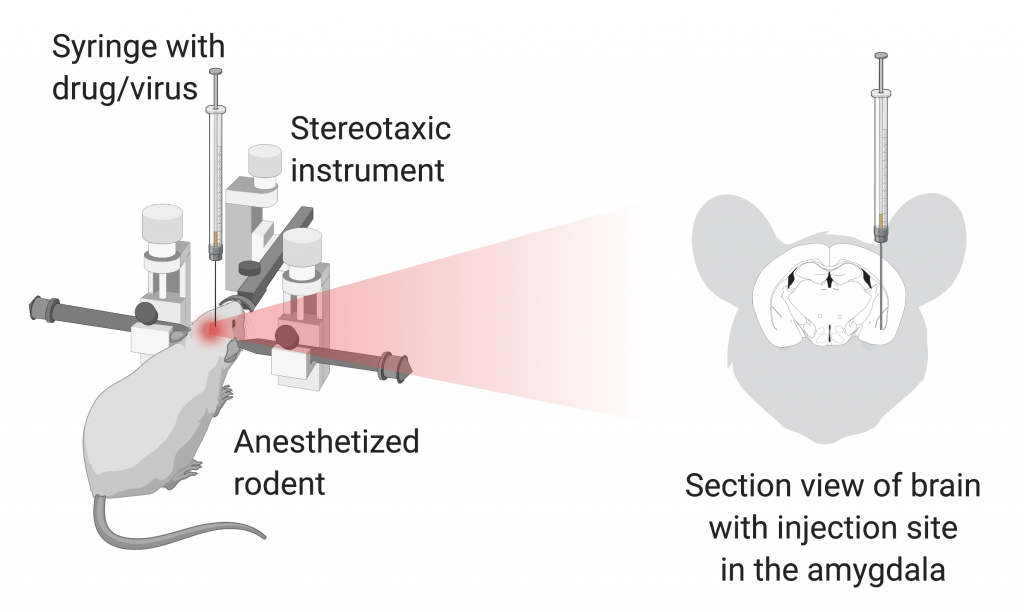
In order to study the amygdala’s role in fear extinction, scientists can inject different drugs into it with very fine syringes (in a procedure called stereotaxic surgery, Fig. 2). By either activating or inhibiting different signaling pathways, they can elucidate what roles those molecules play in the fear extinction process. In addition, experiences like stress and trauma can interfere with this extinction learning, as evidenced in people who suffer from PTSD and in rodent models exposed to different stressful situations, both acute and chronic.
Made with BioRender.
This paradigm has been used by many to study metaplasticity, where the change that occurs is not a modification of the baseline response but rather of the response to a subsequent plasticity-inducing stimulation. For example, Dr. Saha herself showed that it is possible to alter fear extinction learning by injecting a virus into a subregion of the amygdala that disrupts inhibitory synapses. Importantly, this happened without modifying the initial fear conditioning or the anxiety level of the animals. In addition, they also showed that those alterations in inhibitory synapses in the amygdala led to independent changes in the medial prefrontal cortex, hindering its intrinsic plasticity. The same intervention caused increased resilience to acute trauma and improved the performance of a task dependent on another brain region, the hippocampus. Hence, a very targeted intervention in the amygdala can cause an array of effects across multiple brain areas.
This body of research has tremendous implications in our understanding of the brain and how to treat its diseases. On a very pragmatic sense, it should serve as a cautionary tale for researchers to take into account and consider the potential for “undesired” plasticity in more than one place as a response to certain interventions. But more importantly, it opens up potential therapeutic strategies for trauma-related disorders like PTSD, stress or fear. Changes in one small region can lead to widespread effects through its connections to other brain areas. Hopefully, we are a little bit closer to tricking the brain into equating those traumatic memories with what you ate last Tuesday.
Dr. Rinki Saha is a Postdoctoral Research Fellow in the Department of Psychiatry researching stress, and one of CUPS’ social media managers.