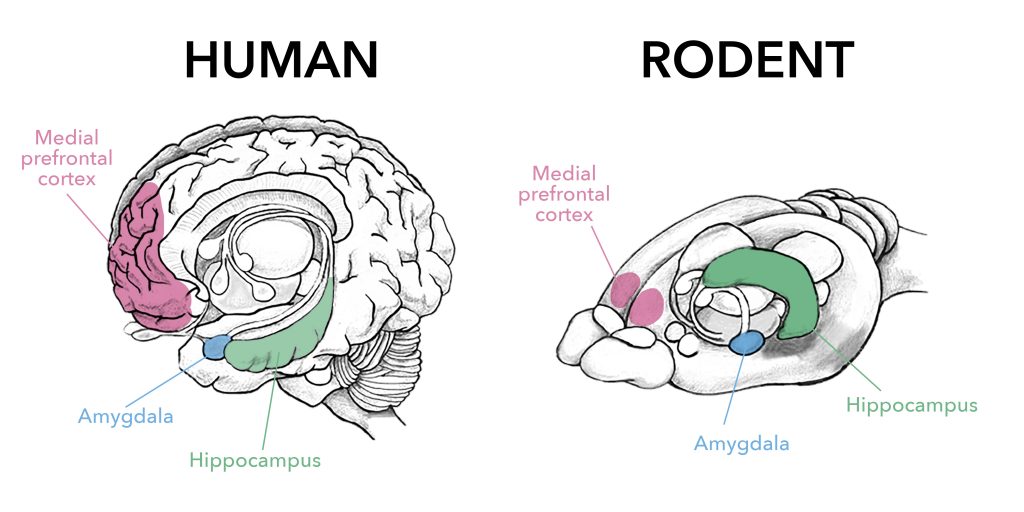
Have you ever wondered why we feel comfortable in a familiar place or why going back to our favorite spots over and over again feels so good? Well, Dr. Paolo Botta, a former postdoc at Columbia University, and colleagues attempted to unravel some of the inner workings of the brain when it comes to rest and exploration. More specifically, Dr. Botta examined how neuronal activity correlates with periods of rest when exploring new areas. Dr. Botta and colleagues followed the behavior of mice as they freely explored a new area. They specifically looked at where and how often these mice decided to exhibit arrest behavior, or, in other words, take a break during their explorations. While the arrest behavior alone is a fascinating phenomenon and provides insight into how mice explore new spaces, Dr. Botta and colleagues decided to go a step further and see which neurons in the brain are important for this arrest behavior. They decide to home in on an area of the brain called the Nucleus of the Basal Lateral Amygdala (BLA). This area has previously been shown to be involved in locomotor exploration, experience based learning, recognition of familiar areas.
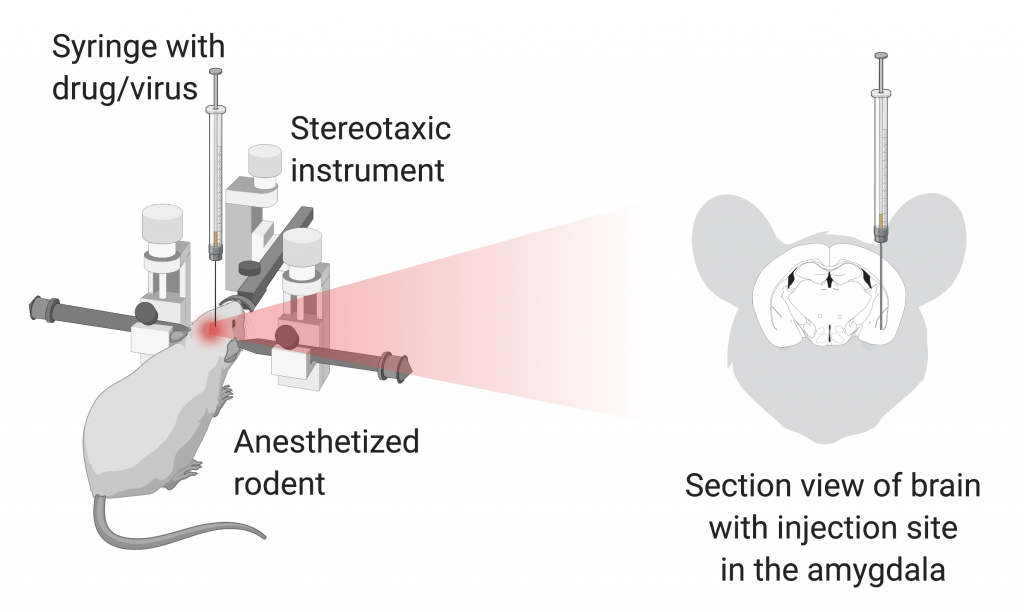
With this information in hand, Dr. Botta and colleagues began by identifying whether BLA neurons are active during arrest behavior. To this end, they gave mice access to both their home cages and a large open area for five days and allowed them to freely explore the large open area during this period. BLA neuronal activity was monitored in the mice by measuring calcium levels, with higher calcium levels indicating neuronal activity (Figure). The researchers observed an increase in calcium in BLA neurons during arrest behavior, which means that BLA neurons are involved in this type of behavior. However, do these neurons actually cause the arrest behavior? To answer this question, Dr. Botta and colleagues either activated or inhibited the neurons using optogenetics. Optogenetics is a technique in which neurons are stimulated by light. So, by turning different lights on and off, the researchers were able to either activate or inhibit BLA neurons whenever they wanted to. When they activated the BLA neurons, the mice decreased their speed and experienced more arrest behavior. When they inhibited the BLA neurons, the mice had an increase in movement speed. After seeing how turning BLA neurons on and off affected behavior, they concluded that the BLA neurons are important for inducing arrest behavior.
At this point, Dr. Botta and colleagues have revealed that BLA neuronal activity occurs specifically during these arrest behaviors and that their activity is important for the onset of the arrest. However, their curiosity did not stop there. They began to wonder whether BLA activity changed when the mice exhibited arrest behavior, or took breaks in more familiar areas. To figure this out, Dr. Botto and colleagues tracked exactly where the mice explored and counted how many times the mice exhibited arrest behavior in areas that they previously explored. With this experiment, they realized that the mice were more likely to exhibit arrest behavior in areas previously visited. So, mice, like humans, have favorite spots and they like to rest in those spots! After seeing that the mice have favorite spots, Dr. Botto and colleagues went on to examine the BLA neuronal activity in these familiar areas. They found that there was an increase in neuronal activity in these familiar areas and the more a mouse revisited and exhibited arrest behavior in a specific area the more neuronal activity developed. In other words, the more often a mouse took a break in a specific area the more that correlated with BLA neuronal activity.
The amygdala has multiple nuclei, which consist of groups of cells that are important for specific roles. The Central Nucleus of the Amygdala (CEA) is a part of the amygdala that has previously been shown to be involved in immobility. BLA neurons also communicate with the CEA (Figure). Knowing that the BLA neurons are important for invoking arrest behavior and the CEA plays a role in immobility, Dr. Botta and colleagues were curious as to whether these BLA neurons that project to the CEA are the specific neurons involved in triggering arrest behavior. To see whether the BLA neurons that project to the CEA are the ones active during arrest behavior they used the combination of calcium imaging and optogenetic techniques previously mentioned. With these techniques they were able to see that the BLA neurons that project to the CEA had an increase in neuronal activity during arrest behavior (Figure). This increase was not seen in BLA neurons that projected to other parts of the amygdala indicating that the BLA-CEA interaction is integral for the arrest activity. They also repeated the stimulation of the BLA neurons that project to CEA and observed an increase in arrest while inhibiting the same neurons resulted in an increase in movement, further confirming the need of this BLA-CEA interaction to induce arrest behavior.
Overall, Dr. Botto and colleagues discovered that BLA neurons that communicate with the CEA are important for arrest behavior, particularly in familiar places. This behavior seems to be extremely important for allowing a mouse to orient itself and properly explore novel surroundings. Maybe humans have a similar pathway that we use when wandering around. Could my BLA be the reason why I always go to the same cafes after a long walk or stop in the same part of the park while walking my dog? Are our BLA neurons just firing away while we rest?

Figure: BLA neuronal activity during exploratory vs arrest behavior. Left: Decreased activity in BLA neurons that communicate with the CEA results in increased exploratory behavior. Right: Increased BLA to CEA neuronal activity, indicated by calcium signaling, results in increased arrest behavior. Red colors indicate decreased BLA neuronal activity and increased exploratory behavior. Green colors indicate increased BLA neuronal activity and increased arrest behavior. BLA: Nucleus of the Basolateral Amygdala, CEA: Central Nucleus of the Amygdala