
Science Stories: Shining light on the neurons that make you move
Author: Marie Labouesse, Post-doctoral Research Fellow in the Department of Psychiatry. Marie studies the neuronal circuits that regulate movement deep inside the brain
Parkinson’s disease is a devastating neurological disorder characterized by severe impairments in motor control. One of the key pathological features of Parkinson’s disease is the gradual cell death of dopamine neurons deep inside the brain. However, dopamine neurons are not the only actors in the picture. In this short story, you will learn about how dopamine neurons communicate through far reaching axon “bridges” with other neurons at the front of the brain to control movement in a coordinated matter.
“Hey Julie, I’ve always wondered why exactly do the hands of our neighbor shake like that? You study this in lab, right?” asked Tom to Julie on their way to work, as they were crossing Roberto in the street. Tom and Julie were both postdocs, but Tom studied plant biology while Julie was a neuroscientist studying animal models of Parkinson’s disease.
“Well- I don’t exactly study Parkinson’s disease (PD), Tom. But I can tell you what I know. Patients with PD have different symptoms, one of which is called tremor, like Roberto having shaky hands. Another major symptom is called bradykinesia, which refers to the inability to start basic movements, for example walking or tying up shoe laces.
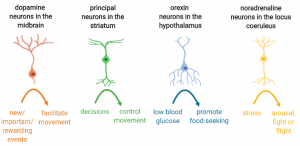
All this is due to a problem happening deep inside the brain. The brain contains about 200 billion cells, 100 billion of which are neurons. Each of these neurons has their own hardrive – the nucleus – that dictates their role in the brain. First, they will each get activated by specific signals: some of them light up if you are hungry or stressed, others get activated by rewarding events or by decisions coming down from the cortex. In turn, neurons will trigger specific responses, like promote movement, help you remember things or help you find food.”
“So which ones are damaged in Roberto’s brain? The ones that make you move?” asked Tom.
“In fact no, the so-called movement neurons are still there in Roberto’s brain. Another type of neuron, known as dopamine neurons, are the ones that die in PD. Dopamine neurons get activated when something new and unexpected is happening, or something rewarding or important. They basically help you pay attention or learn what is worth your time and energy, and in turn they facilitate movements. For example, if your bus is arriving unexpectedly early, your dopamine neurons will light up immediately and help facilitate you running towards the bus. Basically think of dopamine neurons as the steam of your engine, while movement neurons are the wheels of your car, both are crucial.
“So are you trying to find a cure for this?” said Tom.
“No, I’m more in the basic science side of things, I try to understand how the system works when everything is going right. Hopefully it will help other scientists understand what happens when the system is broken and how you might be able to fix things.”
“OK. That makes sense, but then what do you study?” asked Tom.
“You want the full story? So, you see, in PD, dopamine neurons slowly undergo cell death. We don’t fully know why- other scientists are working on this.
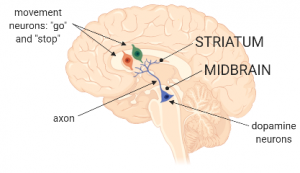
Dopamine neurons are found more or less half-way between your eyes and the back of your head. We call this the midbrain, said Julie. They are also very long. Of course their central part, the nucleus and cytosol, remain in the midbrain. However, to send information to other neurons at distant locations, they use axons which are kind of like highway bridges between two islands. Just like the Brooklyn Bridge between Manhattan and Brooklyn.”
“Which other neurons do the dopamine-neurons talk to in front of the brain, then? Is it the movement neurons?”, said Tom.
“Yes, the movement neurons, exactly. Dopamine neurons in the midbrain communicate with movement neurons that live all the way in the front of the brain – in an area called the striatum. As we said, dopamine neurons get lit up by new or important events, say you’re playing basketball, and someone passes the ball over to you. In response to this event, dopamine neurons are activated and start firing. This basically means there is suddenly a flow of electric charges throughout the neuron’s membranes, all the way throughout the axon. We call this particular flow of electric charges an action potential.
It’s similar to an electric circuit where you’d activate the switch and electrons start circulating. In a neuron, the electric charges can reach all the way throughout the axon and into the axon’s endfeet, also known as synapses. At the synapse, several cellular processes will then be activated by the arrival of these electric charges or action potential. These processes are “electrically-dependent”, just like you’ll need electricity to turn on a lamp that lies in your electric circuit. One of these key synaptic processes is called synaptic transmission, which basically means chemicals will be released from the synapse in the presence of electric charges, and in turn these chemicals can now transmit information to other neurons lying nearby! These chemicals are rightly so called “neurotransmitters”.

The type of neurotransmitter will depend on the neuron’s identity. For example in dopamine neurons, synapses release primarily dopamine. And this is the key: movement neurons in the striatum express receptors for dopamine! So when dopamine neurons release dopamine, this will activate movement neurons. In turn, movement neurons will hand over the message to a set of other neurons through a very specialized circuit. Eventually this circuit transmits the signal directly to the muscles in your arms and instruct them to move.”
“Ok this makes sense. So Roberto’s movement neurons and all the circuit downstream is still functional, right? So why is he not able to control his movements then?
“This is where it gets a bit complicated: Roberto does have the movement neurons as well as the circuits downstream of that. As you said, this works more or less OK (although this is also debatable, but that story is for another day). The main problem to keep in mind is that the message from dopamine neurons is not being properly transmitted to movement neurons. Actually, let me step back for a bit. There are actually not just one, but actually two types of movement neurons: the first type are called #go neurons, and the second type are called #stop neurons. So when dopamine neurons send their message out to #go and #stop neurons, they are going to have an opposite effect on them. It’s kind of like when I use my forward and my brake pedals in the car. I am doing the same movement with my feet, a.k.a. I press a pedal. But when I press the forward pedal, my car will move forward, whereas when I press the brake pedal, I stop!
Same here: when dopamine neurons transfer their message to #go neurons, this will promote movement (as I told you above), whereas when dopamine neurons transfer their message to #stop neurons, this inhibits movement.”
“Ok, so we basically turn on #go neurons when we want to start moving and #stop neurons when we want to pause?”
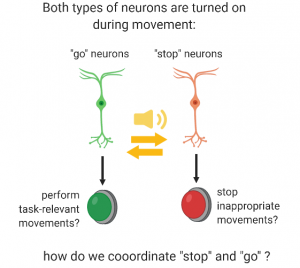
“Well, that’s what we all thought happened until about 10 years ago, so you are pretty close to the truth! But now scientists think it’s actually more complicated than that. These #go and #stop neurons can actually receive messages from many other neurons, in particular the cortex, i.e. the master-controller of your brain. Recent work has shown that when you start moving, both #go and #stop neurons are actually lit up at the same time. This idea was quite revolutionary when it first arrived. One of the current hypothesis is that #go neurons might help you to accomplish the movements you want to do, for example moving your feet in the direction of the bus. On the other hand, #stop neurons will block the movements that are unnecessary, like starting to tie your shoe lace at the same time. This might actually be at the level of more refined movements, like moving your right leg forward, but pausing your left one for a few milliseconds, in order to perform the basic movement of walking.
“What if I am tying my shoes, and then the bus arrives. How do #go neurons and #stop neurons synchronize themselves to switch from one task to the next? Like, how do I not trip myself over?!”, asked Tom, perplex.
“Well that’s exactly what I am studying: it seems like #go and #stop neurons are able to communicate to make sure they are on the same page and that all movements are made in harmony! However, we don’t know yet the full mechanisms by which they communicate or what other partners are involved, that’s what I am looking into.”
“Ok- nice, and how exactly do you study this?”, asked Tom.
“I use two main approaches. The first one is called optogenetics, it was invented only about 15 years ago. It’s a ground-breaking technique that allows to activate or shut off specific neural populations with light. In my case, I want to activate movement neurons. To do that, I use light-sensitive molecules that allow electric charges to enter neurons when exposed to light. Remember, changes in electric charges can promote synaptic transmission and allow neurons to perform their function. Importantly, I can insert these light-sensitive molecules specifically into #go or #stop movement neurons using genetic tools and specific mouse models. And then it’s amazing, I can have mice running around in motor behavioral tasks and once I shine light deep into the brain, I will be able to activate movement neurons at specific moments in the task, allowing me to determine the effects of #go or #stop neuron activation on movement.
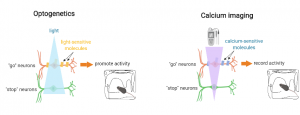
The other approach is known as calcium imaging. It allows me to record the activity of neurons online while mice are performing motor tasks. The reason for this is that when neurons are active, levels of calcium within the cells change at high speed. Tracking calcium levels is therefore a good method to follow the activity of neurons. Technically-speaking, it is similar than optogenetics, except the molecules used will be sensitive to calcium, rather than light. Thanks to calcium imaging, I am able to see how #go and #stop neurons coordinate their activities live during movement.”
“Sounds very exciting. So how will this eventually help Roberto?”, asked Tom.
“Great question. Well the idea is that if we understand the circuits well, we can then go ahead and design better treatments which are more specific. For example, one treatment currently used to help people dealing with Parkinson’s disease is called Deep Brain Stimulation, where patients receive electrical stimulation of a brain area close to the midbrain. If we understand how #go and #stop neurons talk to each other and where exactly in the brain, then we could find new targets for Deep Brain Stimulation to make it work better.”
Images were created on Biorender