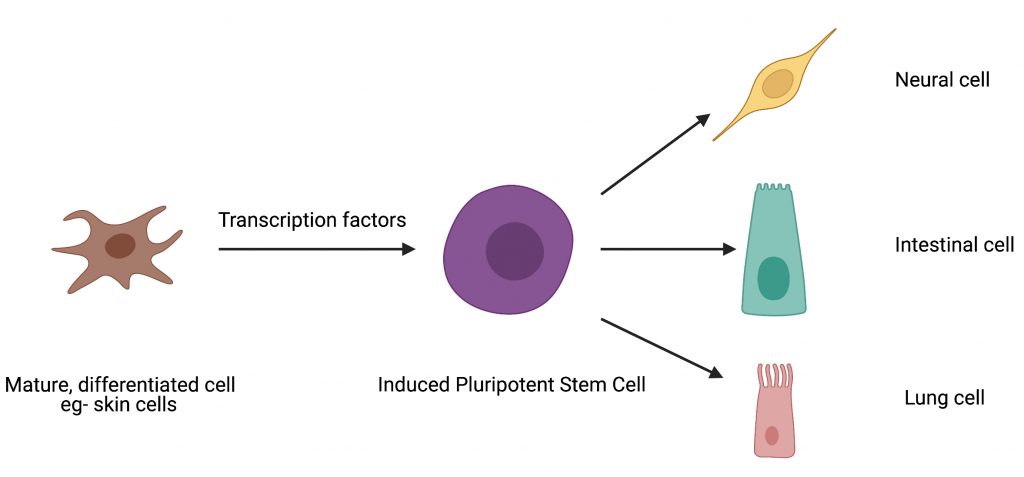
The human body has approximately 15 trillion cells, all of which arise from embryonic stem cells which are considered the building blocks of life. Stem cells renew themselves by dividing indefinitely and can also give rise to cells with specialized functions which ultimately end up forming various organs and tissues in our body. This process is called differentiation. Typically, once cells specialize or differentiate, they lose the ability within the body to go back to being stem cells. Given their unique properties, stem cells have become a critical starting point that scientists can tinker with to develop new drugs and therapies. Because of their tremendous value for research, scientists have figured out non-invasive ways to transform differentiated cells into cells with stem cell like properties. These lab-grown cells, called induced pluripotent stem cells or iPSCs, are typically generated by a process called “cellular reprogramming”.
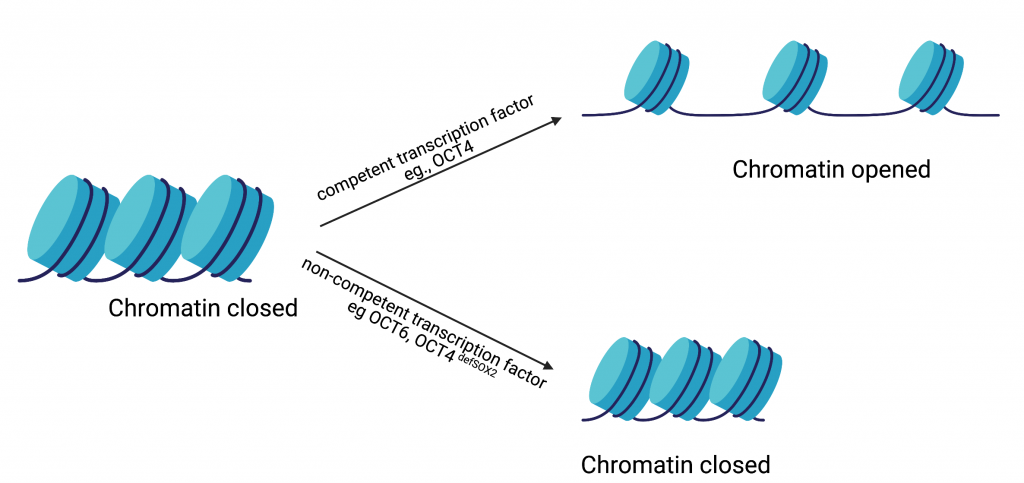
As Dr. Tania Thimraj explains in a recent article, proteins called transcription factors can act as cellular “fixer-uppers” and renovate differentiated cells to look and behave like stem cells. The current state of the art process for making iPSCs involves excess production, also known as overexpression, of the following transcription factors in differentiated cells: Oct4, Sox2, Klf4, and c-Myc (collectively called the “OKSM” cocktail). Despite significant advances in the formulation of this cocktail, there is still a huge margin for improvement in the ability of this cocktail to transform differentiated cells into stem cells. In a recent study performed by Dr. Tan and co-authored by Dr. Malik, the authors propose that the cocktail is not especially effective because the transcription factors were never under any evolutionary selection pressure to produce stem cells. Inspired by this, the authors set out to use evolution in the dish, also known as directed evolution, to make a more efficacious transcription factor cocktail.
Although natural evolution takes place over millions of years, smaller scale evolution can be done in a laboratory setting at much faster timescales. This approach is known as “directed evolution” and has been successfully used by scientists to evolve proteins with new functionalities. This process involves making random mutations in the protein of interest. Then, these mutants undergo a selection process in an appropriate cellular context so that protein variants with desirable properties can be isolated.
In a pioneering study, members of the Jauch lab, including Dr. Malik, used directed evolution to optimize the cellular reprogramming ability of the transcription factor Sox2. Building on this success, the Jauch lab used directed evolution to make ePOU, an enhanced and evolved version of Oct4 which is an integral part of the OKSM cocktail. In the current study for creating ePOU, the authors made random mutations at six functionally important positions in Oct4 and overexpressed the mutant proteins in mammalian cells such that the Oct4 transcription factor activity was tied to the production of a green fluorescent protein representing stem cell transformation.
This innovative study demonstrates that the transformation potential of naturally occurring transcription factors can be drastically enhanced by directed evolution. In addition, this work also provides a framework for future research on transcription factor engineering for cell reprogramming. By providing a faster and more efficient way to produce stem cells, this study has the potential to accelerate various research and therapeutic avenues such as regenerative medicine, drug efficacy and safety testing, and studying human development and disease.
Dr. Vikas Malik is a Postdoctoral Research Fellow in Dr. Jianlong Wang’s lab in the Department of Medicine at Columbia University Medical Center and is a member of CUPS and the Outreach and Communications Committee.