Immunotherapy, a groundbreaking approach to treating diseases by harnessing the body’s immune system, has emerged as a cornerstone in cancer treatment. In contrast to chemotherapy, immunotherapy offers a less toxic alternative for cancer patients. Chemotherapy indiscriminately targets both cancerous and healthy cells throughout the body, whereas immunotherapy specifically focuses on attacking cancer cells. However, the challenge lies in the rarity of responses to immunotherapeutic agents, their limited efficacy across tumor types, and the unpredictable nature of outcomes.
Tumor-infiltrating lymphocytes (TILs), immune cells present in and around tumors, signify an active immune response against cancer. The presence of TILs often correlates with improved patient outcomes, making the identification of predictive biomarkers a crucial pursuit in tumor immunology. Yet, achieving this goal remains a formidable challenge.
Key players in the immune system are white blood cells, produced in the bone marrow, patrolling the body for foreign invaders. Lymphocytes (T cells, B cells, and Natural Killer cells), neutrophils, and monocytes/macrophages constitute the most common types of immune cells.
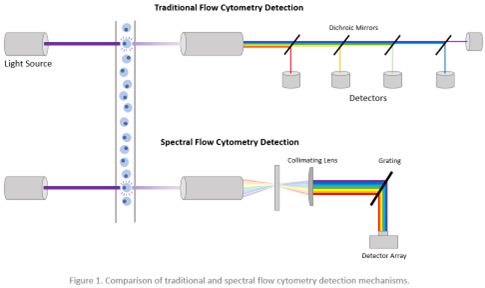
To address the need for biomarkers to guide immunotherapy, a research group led by Dr. Benjamin Izar at Columbia University Medical Center has pioneered a cutting-edge approach. They developed a 34-parameter spectral flow cytometry panel and an advanced data analysis pipeline to explore protein-level immune phenotypes across different cancer phases. Conventional flow cytometry uses dichroic mirrors and band pass filters to select specific bands of the optical spectrum for detection using point detectors. Unlike conventional flow cytometry, spectral flow cytometry captures the entire spectral profile of fluorophores, allowing for a more comprehensive understanding of immune responses (Figure 1). This innovative method enhances signal resolution by subtracting cellular autofluorescence, showcasing its potential to uncover crucial insights into cancer immunotherapy. This allows the use of more existing fluorophores that would otherwise be incompatible on a conventional flow cytometer and the expansion of immunophenotyping panels beyond 40 fluorescent parameters.
In this groundbreaking study, researchers meticulously profiled various tissues affected by prostate and colorectal cancers in mice undergoing anti-PD-1 immunotherapy. Their investigation revealed a significant correlation between the expression of KLRG1, recognized as a coinhibitory receptor on T cells and NK cells, and tumor-related factors such as burden, progression, and regression in response to anti-PD-1 treatment. KLRG1 emerged as a potential marker indicative of terminal differentiation and/or senescence, influencing key cellular pathways and checkpoints.
The longitudinal high-parameter spectral flow cytometry approach employed in this research showcased its prowess in extracting novel targets and biomarkers from dynamic ‘temporal atlases’ of antitumor immunity. This innovative methodology holds the promise of unraveling the intricate, ever-changing interactions within the tumor microenvironment. By providing a deeper understanding, it aims to enhance the efficacy of immunotherapy in clinical applications. The study particularly emphasizes the need for further exploration of KLRG1+ CD4 T cell subsets as potential targets or prospective biomarkers for advancing cancer immunotherapy.
For more details on this research, refer to the article: “KLRG1 Marks Tumor-Infiltrating CD4 T Cell Subsets Associated with Tumor Progression and Immunotherapy Response.”
Written by: Trang Nguyen
Reviewed by: Erin Cullen