Self-renewing stem cells are capable of developing into certain specialized cell types thus making them ideal candidates to study human development and as potential treatment modalities for a range of diseases. There are three types of stem cells: embryonic stem cells, adult stem cells and induced pluripotent stem cells. As the name suggests, embryonic stem cells are found in the embryo at very early stages of development. Adult stem cells are found in specific tissues post development. However, using human embryonic stem cells in research is quite restricted due to ethical, religious, and political reasons. This limitation has resulted in the identification of cell reprogramming techniques to convert differentiated cells, such as skin cells, back to an embryonic stem cell state through a process called induced pluripotency. The resulting induced pluripotent stem cells (iPSCs) are equivalent to the natural human embryonic stem cells and can be differentiated to any desired cell type using a mixture of biological molecules.
Cell reprogramming techniques can be likened to fixer-uppers. Imagine trying to remodel a building for a different purpose – converting an office building into a residential one for instance. Though the building material can be reused, with the aid of experts, there would be some structural changes and remodeling necessary to make it a home. Similarly, cellular reprogramming is the technique by which one cell type can be converted to another cell type in the lab with the help of certain gene expression regulators called transcription factors (Fig. 1). The process of inducing pluripotency has been studied extensively and the overexpression of four transcription factors – OCT4, SOX2, KLF4, cMYC (collectively referred to as “OSKM”) – has been shown to induce pluripotency in mouse skin cells.
Many studies have tried to identify other transcription factors with the potential to induce pluripotency or to replace OSKM in an effort to enhance the efficiency of iPSC generation. Of these four transcription factors, SOX2, KLF4 and cMYC have been successfully replaced by members of their protein family to induce pluripotency. However, replacing OCT4 with structurally similar and evolutionarily related factors failed to show similar reprogramming capabilities. This could indicate the presence of special molecular features on OCT4 that give it the ability to reprogram cells. However, these special features and the molecular mechanisms that enable OCT4 to induce pluripotency remain to be identified.
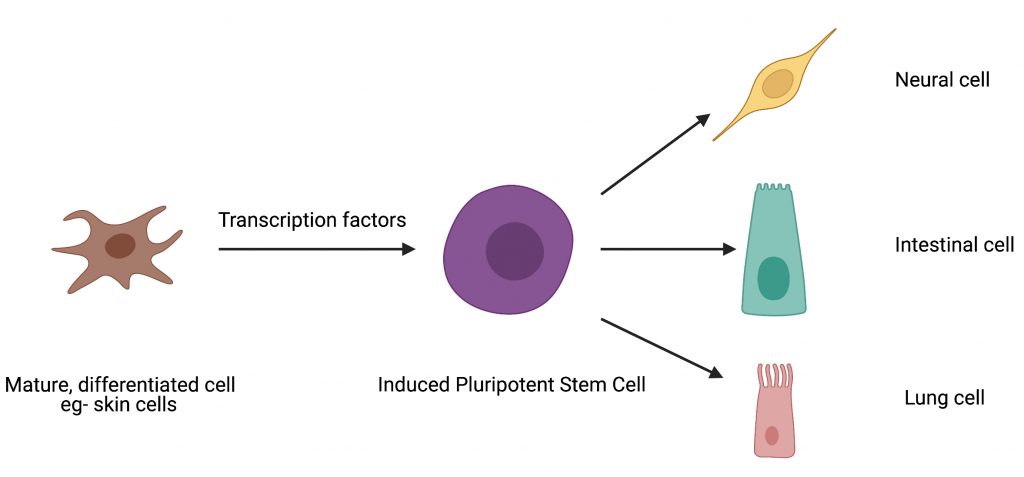
In the current study, Dr. Malik and colleagues hypothesized that the ability of a transcription factor to reconfigure chromatin (the complex of macromolecules composed of DNA, RNA, and protein, which is found inside the nucleus of eukaryotic cells), is one of the features that distinguishes a reprogramming competent transcription factor from a non-competent one (Fig. 2). To test this hypothesis, they studied the well-established OCT4-SOX2 relationship from initiation to maintenance of pluripotency. They performed their study by comparing DNA-accessibility, DNA-binding, and transcriptional control by OCT4, OCT6 and an OCT4 mutant that does not interact with SOX2 (OCT4defSOX2) during early, mid and late phases of cell reprogramming. What makes this study particularly interesting is the fact that a previous study by the same group has shown that OCT4 naturally interacts with SOX2 to induce pluripotency, whereas OCT6 can only induce pluripotency when OCT6 was mutated to enhance its interaction with SOX2. Dr. Malik’s current study focuses on the mechanisms by which the above-mentioned transcription factors interact with chromatin and in turn bind to the transcription factor binding sites on the genes that are involved in processes from the initiation to maintenance of induced pluripotency.
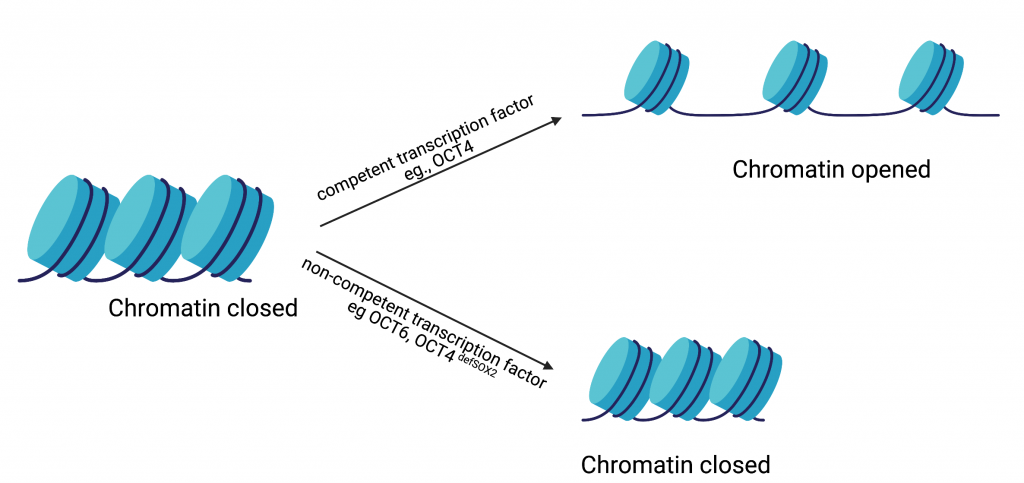
From this study, the researchers found that OCT4, OCT6 and OCT4defSOX2 have unique transcription factor binding sites on the pluripotency-related genes which could explain why substituting OCT4 with related transcription factors does not activate these genes. The results from this study challenge previously established roles for OCT4 in driving pluripotency. Dr. Malik and colleagues have identified distinct modes of chromatin interaction and roles for SOX2 and OCT4 during initiation, progression and maintenance of pluripotency. They found SOX2 to be a better facilitator of chromatin opening and initiator of pluripotency compared to OCT4. Once the cells have been initiated towards pluripotency, OCT4-SOX2 binding is required to see the process through and once the cells are pluripotent OCT4-SOX2 binding becomes less essential. The most important role of OCT4, they found, was to maintain the cells in a pluripotent state as opposed to its previously investigated role as an initiator of pluripotency.
The results from this study contribute new insights to a rapidly progressing field. Identifying the roles of key factors during the stages of reprogramming would add vital pieces of information to the big puzzle of cellular reprogramming. These pieces of information would considerably enhance the use of stem cells as potential therapeutic candidates for a number of diseases .
Dr. Vikas Malik is a Postdoctoral Research Fellow in Dr. Jianlong Wang’s lab in the Department of Medicine at Columbia University Medical Center and is a member of CUPS and the Outreach and Communications Committee.